Just recorded a video on how you can view a SAS dataset on an iPhone. http://bit.ly/rHJbX
Posts
Showing posts from August, 2009
My SAS Macro Ran with ERRORs on iPhone. The Beauty of SAS Log
- Get link
- Other Apps
SAS macros are script files that execute SAS instructions similar to other SAS programs. It is commonly used to automate tasks since the macros can perform repeated tasks with parameters that can be selected by the user. On the iPhone, this can appear as a list screen with selection choices so the macro can be performed on the server from the selected values on the iPhone. The SAS programmer that loads the macro on the server will configure the macros with different type of parameter selection type including the following examples: On Off Selector These are similar to check boxes where it can be set to on (checked) or off (unchecked). Short Text and Passwords The single text entry allows users to enter any text value to the entry. A similar entry type is the password which will mask the entry so that the text is hidden. Check List This include a check list where the user can select one value from a list of valid values. Tex
How do you view SAS datasets on an iPhone? You chunk it.
- Get link
- Other Apps
Data Overview SAS datasets are stored in libraries on the server similar to how files are stored in folders. Each library is associated with a different folder on the server which contains one or more datasets . You then have the option of selecting the library which contains datasets you wish to view. The screen that lists the available datasets can be accessed through the " Dataset " navigational button at the bottom of your screen. This will list all the available SAS dataset from the specified library. You can then view the contents of the data by selecting on dataset from the list. This is accomplished by tapping anywhere on the row of the displayed dataset name. Selecting Data The main dataset screen displays all datasets that you have access to in the specified library. The list displays a short dataset name followed by the more descriptive dataset label below each dataset . You can select a different library by selecting the library name at the top of the
Most Overlooked Variable Length Error for SAS CDISC Data
- Get link
- Other Apps
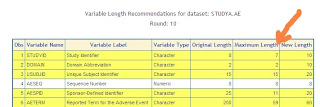
The FDA review of an electronic submission requires the merging of submitted data to confirm that the source produces the same aggregate results of the submitted summary analysis. This can only be accomplished if there are clearly defined keys between the datasets and that the keys have standard attributes. A common error that would occur is that the length of the key fields is slightly different. For example, the study identifier (STUDYID) of one set of data is set to length of 7 and another is set to 10. When the two sets are merged, some of the variable values will be truncated leading to errors. An evaluation if key field lengths are crucial in standardizing the key field lengths. CDISC standards are very helpful in getting the variable attributes such as names and labels standardized. It however, does not enforce the standards of lengths leaving it up to you to evaluate and come up with the correct length for each study submitted. The following steps are recommended to standard yo
The Simplest SAS PROCs Can Verify CDISC Transformations
- Get link
- Other Apps
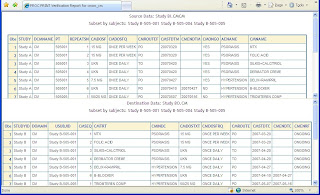
Clinical data that has been originally captured from case report forms and then transformed into CDISC SDTM format requires rigorous verification and validation. This will ensure that it meets the guidelines data structure and that the clinical data that has been transformed has not been affected during the transformation. I will recommend a series of steps that uses very basic SAS procedures including PROC PRINT, PROC FREQ and PROC MEANS that will assist you in this validation endeavor. Step 1 – Print Sample You can create a PROC PRINT of a subset of just three subjects. This can then be visually reviewed to make sure there are no major changes. Step 2 – Frequency Counts For variables that have a small set of distinct values or otherwise known as categorical data, a PROC FREQ is useful for verify if the summary counts between the source and destination matches. Step 3 – Means Statistics For numeric variables with lots of values or otherwise known as continuous data, a PROC MEANS can v
When are CDISC Datasets Useful for ISS?
- Get link
- Other Apps
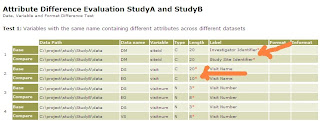
One important objective in converting to CDISC standards is to then gain the ability to perform an ISS (integrated safety summary) analysis between multiple studies. Once the data is created in a standard format, it makes it easier to merge the data from a pool of studies since they are in the same CDISC structure. Even though the variables names and labels are standardize, the guideline does not strictly specify the length and other detail attributes. In a recent set of studies I was working on, we ran into discrepancies between two studies even though it was converted to CDISC . The following report illustrates this problem. Most of the issues came about due to length differences between the two studies. This can lead to truncation if any of these key fields are merged. Other less common issues are things such as labels being different. This can be due to using different versions of the guidelines such as 3.1.1 versus 3.1.2. I ran a % difftest macro which then revealed some diffe
Post SAS Data on Facebook Wall
- Get link
- Other Apps
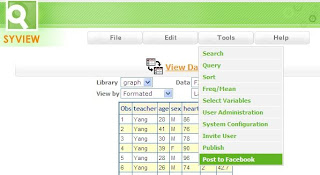
In the age of Google, information is liberated and readily avialable. However, SAS data is often locked behind servers limited to analysts and statisticians. Facebook is the most popuplar social network and is a common way how users share information. I will show you how you can share SAS dataset on Facebook by doing the following steps. View Data - You would view your SAS datasets as before with Syview. Select the view which best represent your data. Post to Facebook - From the data view on Syview , select the menu: Tools > Post to Facebook Note that a thumbnail view will be captured of the current view. Write Something - When posting things on Facebook walls, it is recommended that you write a little short message. This describe to your reader what this is about. View Posting - Log onto Facebook and view the data posted. Note that a thumbnail image of the data view is created along with the message you typed. Users can click on the thumbnail for detail view. Detail Data View -