The Simplest SAS PROCs Can Verify CDISC Transformations
Clinical data that has been originally captured from case report forms and then transformed into CDISC SDTM format requires rigorous verification and validation. This will ensure that it meets the guidelines data structure and that the clinical data that has been transformed has not been affected during the transformation. I will recommend a series of steps that uses very basic SAS procedures including PROC PRINT, PROC FREQ and PROC MEANS that will assist you in this validation endeavor.
Step 1 – Print Sample
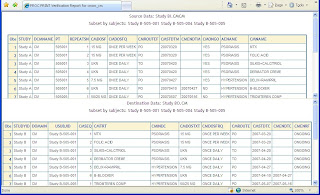
You can create a PROC PRINT of a subset of just three subjects. This can then be visually reviewed to make sure there are no major changes.
Step 2 – Frequency Counts
For variables that have a small set of distinct values or otherwise known as categorical data, a PROC FREQ is useful for verify if the summary counts between the source and destination matches.

Step 3 – Means Statistics
For numeric variables with lots of values or otherwise known as continuous data, a PROC MEANS can verify if the values are the same.

These results were produced from using a SAS macro %verification_rep which generates the reports using SAS PROCs with ODS so that the results are generated in HTML in a frame so that the data can be reviewed side by side. This provides you with a quick visual inspection that can easily identify data differences or discrepancies that are introduced during data transformation to CDISC standards.

Comments
Post a Comment